- Language
- 🇺🇸
- Joined
- Nov 17, 2022
- Messages
- 32
- Reaction score
- 11
- Points
- 8
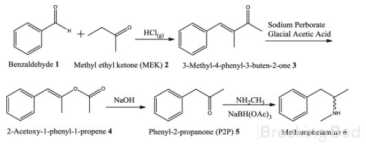
This method looks awesome, otc cheap chemicals.
Why expensive dcm while chloroform exists. If you want a long time coock you ll understand making chloroform is the job you have to do.
The write up method shared here is perfect, it shows it written by a professional . I ll wonder if there are any video a bout this method. Tnx for sharing this nice write up willi
Why expensive dcm while chloroform exists. If you want a long time coock you ll understand making chloroform is the job you have to do.
The write up method shared here is perfect, it shows it written by a professional . I ll wonder if there are any video a bout this method. Tnx for sharing this nice write up willi